TUESDAY, 1 DECEMBER 2020
Solar power is widely considered to be one of the most important renewable power sources, with an industry worldwide worth $183 billion. This figure grows each year as the popularity of solar energy increases, but the use of solar power still needs to increase dramatically if we are to reduce fossil fuel reliance to zero. Hence, it is crucial to improve the efficiency of the solar cells we currently manufacture. This turns out to be quite difficult; conventional silicon solar cells have a predicted limit of only 29% efficiency due to the narrow range of the sun’s light spectrum that they are able to absorb. This stumbling block has encouraged scientists in the last decade to look elsewhere for alternative designs with the potential to reach higher efficiencies. One such alternative is perovskite solar cells which have already reached 25.2% efficiency and have a predicted maximum of 43%. Currently, the lifetime of these devices is a problem, since power output decreases with time due to the perovskite layer’s tendency to slowly degrade, especially in contact with moisture. They can also be cost ineffective, and suffer from scalability and toxicity issues. A lot of research is being dedicated to mitigating these problems, making perovskite solar cells an increasingly viable alternative to our current silicon-based panels. Here, I examine how these cells work and the new possibility of using ion-blocking polymers to improve their lifetime.The promise of perovskites
‘Perovskite’ is a family of materials, with the composition ABX3 in the arrangement shown below, where for solar cell A is an organic cation, B is a metallic cation (typically lead or tin) and C is a halide ion. The process of generating electricity begins with electrons in the perovskite layer of the solar cell absorbing photons of light to create electron-hole pairs (excitons). The photon energy excites the electron to a higher energy state (the conduction band), in which they can move freely through the lattice, leaving an extra positive charge remaining on the original ion (a hole). From here, the electrons are attracted towards the anode of the cell, which usually incorporates electronegative elements like fluorine, and holes move towards the cathode, which is usually made of gold or silver, since they are electron-releasing and inert. A circuit connects the anode and cathode, allowing electrons to flow between the two and recombine with the holes. This current is what makes the solar cell a viable source of energy.
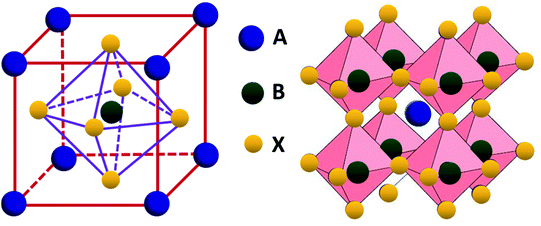 Structure of perovskites with the ABX3 formula - published by the Royal Society of Chemistry at https://pubs.rsc.org/en/content/articlelanding/2019/na/c8na00416a. Image licensed through creative commons.
Structure of perovskites with the ABX3 formula - published by the Royal Society of Chemistry at https://pubs.rsc.org/en/content/articlelanding/2019/na/c8na00416a. Image licensed through creative commons. The major problem with perovskite cells is that their lifetime (how long they produce power at the original high efficiency for) is shorter than conventional silicon cells. Perovskite tends to degrade and lose its perfect crystal structure, either because of the organic A cations decomposing into other by-products, or the lattice breaking up by ionic diffusion along grain boundaries. Both of these changes hinder how well the device absorbs light and transports electrons and holes. The chemical decomposition route can be caused by external stimuli like moisture, which is particularly dangerous as it leads to soluble toxic by-products like lead iodide, though this problem can be mitigated reasonably easily by techniques like encapsulation. However, the second reason, internal ion movement, is more problematic as it is not caused by any external stimuli that we can block. The root of this movement lies in the fact that perovskites are good solid-state conductors because the B ions are only loosely held by the six surrounding X ions. The migration of B ions across the cell leads to accumulation of charge and an undesirable permanent voltage across the cell, and their movement can lead X ions to leave their sites as well, causing loss of the perfect perovskite crystal structure and that of adjacent layers.
Polymer barriers
In October of this year, Sai-Wing Tang, leading a collaboration of researchers from three universities in Hong Kong and Singapore, announced findings of a unique mechanism using polymer barriers to reduce ion migration. Ion migration tends to occur along grain boundaries, which are the interfaces between sections of the crystal that originally grew as unique entities. Ions move along these boundaries because, here, B ions are not fully coordinated (a covalent bond in which both electrons come from X) by six X ions (see illustration below). This means it takes much less energy for B ions to hop between positions and migrate along the boundary. With a polymer incorporated, as the perovskite grains grow and merge together, the polymer chains are left trapped between the grains. There, they act as a physical barrier to B ions leaving their lattice sites and thus significantly slow ion migration.
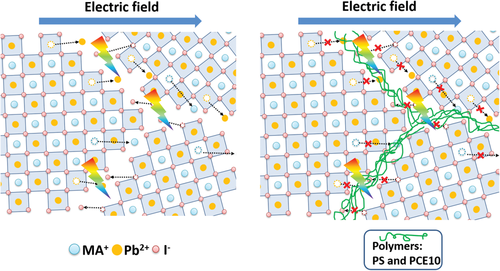 An illustration of ion migration with (right) and without (left) polymer intervention. Extracted from https://onlinelibrary.wiley.com/doi/full/10.1002/adfm.202006802 with permission from John Wiley and Sons.
An illustration of ion migration with (right) and without (left) polymer intervention. Extracted from https://onlinelibrary.wiley.com/doi/full/10.1002/adfm.202006802 with permission from John Wiley and Sons.This new research is highly valuable in demonstrating, through microscopy techniques and mobile ion concentrations, how polymers can be used to block ion migration in perovskite cells to enhance their stability. Going forward, a wider body of research is needed in order to consider how this principle can be made commercially profitable. Notably, the choice of polymer must be carefully assessed because it may influence the perovskite in other ways. For instance, some polymers will be able to act as surfaces for the perovskite to initially nucleate (grow) off, potentially leading to fewer, larger grains. This in turn might lead to less ion migration, since there are fewer grain boundaries to migrate through. Having said this, it seems highly likely that, once further developed, this polymer-based solution will have an excellent commercial market, since perovskite cells are now reaching the stage of being produced in volume. Oxford PV produces perovskite solar cells as part of a tandem unit with silicon solar cells and their new scaled-up production line for this product is due to finish construction at the end of this year.
In light of the limits of current silicon solar cells, devices like perovskite cells with high predicted efficiencies continue to gain increasing attention. From the moment they initially caused great excitement in 2012, when Professor Henry Snaith from Oxford University published his research detailing in Science the unprecedented efficiency of perovskites in solar cells, perovskites have not faded from the spotlight, and considerable research efforts are still ongoing, eight years later, to improve on device efficiency, lifetime and cost. This is an indication in itself towards the potential of the technology. The ion-blocking polymers described here are just one example of such research efforts, and present another important step forward in improving the lifetime of perovskite solar cells. Overall, given the research attention they continue to receive from both universities and start-up companies, and the clear promise of their outstanding predicted efficiency limit of 43%, perovskite solar cells seem to be a technology that is likely to form an important part of future solar energy generation
^ Really the movement of holes is due to that of electrons - this is analogous to a row of five chairs with four people on, when the empty chair starts on the end but once everyone has shifted appears to have moved to the other end.
Pip Knight is a second year student from Churchill College studying Natural Sciences.
