SATURDAY, 7 MAY 2011
It was late January 1951 when a young African-American woman walked into Johns Hopkins Hospital complaining of a “knot on her womb”. Her name was Henrietta Lacks and unbeknownst to her, or her family, this event changed the face of medical and biological research. Her treatment for cervical cancer resulted in the creation of the HeLa cell line, the first immortal human cell line—a major accomplishment in the new and rapidly growing field of cell and tissue culture.Fascination with cells has engaged scientists since the 17th Century, when Robert Hooke looked down a microscope at cork bark and saw the basic building blocks of life for the first time. He named these repeating blocks “cells”. Soon afterwards, in 1674, inspired by Hooke’s work, the Dutchman Anton van Leeuwenhoek observed living and moving, single-celled microbes. By the early 1800s, many scientists including Robert Koch and Louis Pasteur had pioneered the culture and study of microbes.
However, a breakthrough in culturing cells from multicellular organisms would not occur until almost a century later. In 1907 Ross Harrison found himself involved in a debate over whether or not nerve fibres were outgrowths of individual cells. The study of the nervous system had traditionally been purely observational. Harrison decided to resolve the debate by studying nerve fibre growth in vitro. To do this, he adapted the hanging drop technique developed for the culture of microbes. Harrison cut fragments of frog neural tube, the embryonic precursor of the central nervous system, and placed them in a drop of frog lymph fluid on a coverslip. The coverslip was inverted over a glass slide with a well in the middle, allowing the clotted lymph droplet to hang suspended from the coverslip. Within this droplet, Harrison was able to maintain the cells alive in the tissue and observe the active outgrowth of nerve fibres from them—thus settling the debate.
While Harrison’s work had solved the debate, his work would prove of foremost importance in establishing the field of cell culture. Using his skill and training as a surgeon, he had solved the basic problems of culturing tissues: through his choices of growth medium, culture vessel and surgical methods for preventing contamination, he provided scientists with the basic tools for culturing cells and tissues. Harrison was a modest man, and his groundbreaking experiment went largely unnoticed by the public and the media. However the scientific community were very interested, and cell culture developed with great pace. As with any newly emerging field, cell culture was at times surrounded by controversy. This simultaneous furtherance and hindrance of the field is no better embodied than by the French surgeon and biologist, Alexis Carrel, who pursued the work Harrison had started.
After receiving much acclaim for his novel surgical techniques, Carrel turned his attention to cell culture. Under the tutelage of fellow scientist Montrose Burrows at the Rockefeller Institute, Carrel began to culture tissues from many different animals. He used methods that Burrows had learnt while visiting Harrison’s lab, adapting and perfecting these techniques. Progress was fast, and soon Carrel and his colleagues were able to subculture tissues into new plasma clots by carefully cutting the tissues into smaller fragments. In doing so, they created the first cell lines.
 Carrel was awarded the Nobel Prize in 1912. In the same year, he published a controversial paper claiming he had managed to culture an “immortal” cell line from a chicken heart. His Nobel Prize ensured Carrel became an instant celebrity, and although his prize was in recognition of his surgical efforts, much attention was given to Carrel’s cell culture research. It was often misrepresented, and many journalists believed that Carrel had managed to grow an entire immortal chicken heart from these cells. The American public developed a keen interest in his work, believing Carrel had found a way of cheating death. Carrel cultivated his celebrity status, and each year on the 17th January he would gather his lab members, morbidly dressed head to toe in their black gowns—which he wrongly believed protected the cells from light—to sing ‘Happy Birthday’ to the cells. There was scarcely a year when the coming of age of this ‘immortal’ cell line was not reported in a newspaper or magazine, although many scientists remained sceptical and resented his fame.
Carrel was awarded the Nobel Prize in 1912. In the same year, he published a controversial paper claiming he had managed to culture an “immortal” cell line from a chicken heart. His Nobel Prize ensured Carrel became an instant celebrity, and although his prize was in recognition of his surgical efforts, much attention was given to Carrel’s cell culture research. It was often misrepresented, and many journalists believed that Carrel had managed to grow an entire immortal chicken heart from these cells. The American public developed a keen interest in his work, believing Carrel had found a way of cheating death. Carrel cultivated his celebrity status, and each year on the 17th January he would gather his lab members, morbidly dressed head to toe in their black gowns—which he wrongly believed protected the cells from light—to sing ‘Happy Birthday’ to the cells. There was scarcely a year when the coming of age of this ‘immortal’ cell line was not reported in a newspaper or magazine, although many scientists remained sceptical and resented his fame.Carrel’s work incontrovertibly advanced the field of tissue culture. His fame attracted many talented scientists to his lab, even the famous American aviator Charles Lindbergh. Lindbergh was instrumental in designing much early cell culture apparatus thanks to his engineering skills. However, Carrel’s claim of an ‘immortal’ cell line which supposedly lived for 36 years, seems to have been, at best, an experimental oversight or, at worst, an embellishment of the truth. In 1961 Hayflick and Moorhead showed that cells taken from a normal organ have a limited ability to divide and grow; in the case of the chicken heart cells they could only be sustained for 60 to 80 days without actively inducing an event known as transformation, whereby cells become cancerous and able to replicate indefinitely.
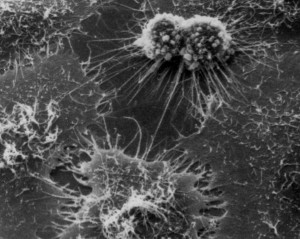
The first irrefutably immortal cell line is accredited to the L cell line derived from a mouse cancer in 1948 by Wilton Earle, a former Carrel lab member. The race was now on to create an immortal human cell line, and George Gey at Johns Hopkins University was determined to be the first. He regularly received tissue samples from African-Americans receiving free treatment for cancer. Up until 1951 he had no success in maintaining any of these cells; Henrietta changed everything. While carrying out an operation on her, the surgeon cut a slice of her malignant cervical tissue and sent it to Gey. Soon afterwards Gey had the first rapidly proliferating human cell line, one that would be in demand from scientists the world over.
Henrietta’s cells have provided unparalleled insight into the biology of human cells. As a consequence of studying these cells, we have a better understanding of what goes wrong in many diseases such as cancer. The use of HeLa cells has also significantly reduced the need for animal experimentation. For example, in 1952 millions of HeLa cells were grown to test the safety and effectiveness of the new polio vaccine, thus avoiding the use of primate testing. HeLa cells have also caused us to consider ethical practice to a greater degree. Henrietta’s cells were taken without her consent, and her family were left unaware of their existence for many years, during which time many people made millions of dollars selling and using HeLa cells. In the mid-1960s, as a direct result of experiments using HeLa cells, stricter rules regarding human experimentation were put in place, with informed consent now a standard requirement in any human study.
Although HeLa cells are still used in many labs today, researchers have since isolated over 3400 different cell lines from 80 species. While scientists have not yet cultivated an ‘immortal heart’, they are now much closer to being able to repair heart tissue. The discovery of stem cells, which have the ability to grow into different tissue types, provides much promise for the treatment of many diseases, from Alzheimer’s to diabetes. The ability to culture stem cells, or indeed any cells, would not have been possible without the pioneering work of these scientists, who through their simple experiments made cell culture what it is today.
Nicola Stead is a PhD student at the Babraham Institute