TUESDAY, 29 APRIL 2014
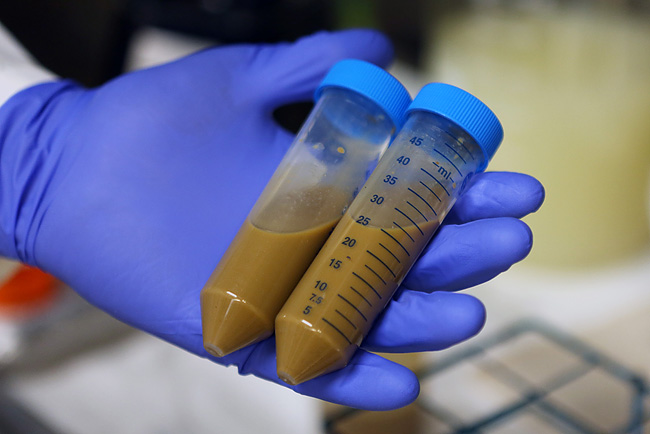
In the UK, about 25,000 people each year are diagnosed with RCDI, and the medical community is scrambling to improve treatment options. The 'faecal microbiota transplant' captures healthy bacteria from donor faeces by blending poop with a liquid and straining before pumping the solution into the patient's gastrointestinal tract through the nose or the colon. While it sounds gross, cure rates are high, though researchers have yet to determine exactly how it works. Doctors with patients seriously ill by C. difficile are conflicted, with some refusing to provide this treatment option while others are eager to try. Regulatory authorities are slowing patient access as they grapple with guidance procedures to ensure high standards of care for this new and unusual treatment. In Canada, an experimental faecal transplant project has been shut down after the national health authority deemed the administration of faeces akin to a drug trial. The ruling considers faecal transplants 'investigative' and requires that researchers seek authorization for full clinical trial.
At the Fraser Health centre in British Columbia, Dr Ed Auersperg was preparing to start a pilot project of faecal transplants until he received notice Friday from Health Canada that it would be shut down.
As reported to the Globe and Mail, Health Canada said, “Since no company or individual has sought market authorizations for materials used in fecal therapy, the therapy is considered investigational, meaning that fecal therapy can only be conducted in the context of an authorized clinical trial.”
Fraser Health's medical director, Dr Elizabeth Brodkin, told the Globe and Mail on Monday that, “We didn’t solicit Health Canada approval for the pilot since we did not consider faecal content would be considered an experimental drug.”
Dr Auersperg believes that by classifying faeces as a drug, Health Canada is preventing patients' access to treatment that may be the best option for fighting RCDI. He said, “How you can take stool and call it a drug, I don’t know. You have no idea how disappointing it will be for people who have been having diarrhea 20 times a day for the last nine months.”
According to the Food and Drug Act, Health Canada considers a drug to include,
"Any substance or mixture of substances manufactured, sold or represented for use in:
1. the diagnosis, treatment, mitigation or prevention of a disease, disorder, abnormal physical state, or the symptoms thereof in man or animal
2. restoring, correcting or modifying organic functions in man or animal,
3. or disinfection in premises in which food is manufactured, prepared or kept."
With that broad definition, one might argue that even a glass of water could be considered a 'drug', but Dr Auersperg admits that, "...I get where they are coming from. They have to ensure patients get standard, modern medical therapy. A fecal transplant is about as far as you can get to the other end of the spectrum.”
There are at least nine clinical trials for faecal transplants currently authorized by Health Canada. While Fraser Health did not seek clinical trial status, they did get permission from the British Columbia College of Physicians and Surgeons because Fraser Health considered the project a health care procedure regulated at the provincial level.
Dr Thomas Louie at the University of Calgary, who is preparing an authorized clinical trial, told the Globe and Mail, "The procedure is advanced enough that whether you do it as a pilot or a trial – you will help patients."
Ironically the Fraser Health study shutdown comes after one of its doctors, Jeanne Keegan-Henry, was featured in their magazine about moving past the barriers to faecal transplants.
In the UK, the National Institute for Health and Care Excellence (NICE) has moved treatment ahead with its March 2014 report on guidelines for what they call the 'faecal microbiota transplant'. In the assessment, NICE reports a cure rate of 94% in a trial of 43 patients, and symptom resolution in 91% of all patients treated based on a summary of 25 studies with 289 participants. In the meta-analysis, only 2% of faecal transplant patients reported a relapse after 29 days to 4 years.
Currently the first line of treatment for RCDI is with the antibiotic Vancomycin, but a recent study in the New England Journal of Medicine showed that faecal transplant was three times more effective. With slow progress by doctors and regulatory agencies to make medical faecal transplants widely accessible, there is a growing 'do it yourself' (DIY) movement, which could pose serious health risks, especially if the donor faeces are not first screened for diseases or pathogens.
This Mosaic article provides a glimpse into the history of faecal medicine and includes a 'DIY' faecal transplant video from YouTube.
To read the full Globe and Mail articles click here and here.