TUESDAY, 20 MAY 2014
Frederick Sanger, the father of the genomic era, died peacefully last November. He left his legacy: a revolutionised field of biology. This article attempts to describe his life, his achievements and their wide-reaching impact.Sanger was born in 1918 into a Quaker home in Gloucestershire. He inherited his father’s love for science and at 18 he gained a scholarship to study Natural Sciences at St John’s College in Cambridge. Struggling in Maths and Physics, he decided to enrol on a newly-founded course: Biochemistry, in which he excelled, graduating in 1939, as war began.
A conscientious objector to battle, he spent the days of conflict in Cambridge carrying out research on nitrogen uptake in root vegetables. In 1943, he continued his studies in Cambridge with a challenging postdoctoral research project investigating protein structure. At the time, the understanding of proteins was in its infancy. People knew they were made of amino acids, but how these were arranged was subject to hot debate. Some thought their arrangement was variable, whereas others, like Sanger, were convinced the order and composition of the amino acids were fixed and unique. When Sanger applied for funding to the Medical Research Council (MRC) to study the sequences of amino acids that make up a protein, his proposal was considered futile and denied. Eventually, he obtained enough smaller grants and began working with one particular protein: insulin.
Insulin is a peptide hormone, secreted by the pancreas, which switches on anabolic, reserve- making metabolism. People who suffer from Type 1 diabetes are unable to produce insulin, and at that time children with this disease would starve to death as their bodies could not utilise nutrients. When this vital protein was discovered in 1921, significant international efforts were made to isolate it, eventually leading to techniques that enabled its separation and purification on a large scale. This gave Sanger the substrate he needed: a pure, cheap and short-chain protein.
Sequencing proteins was not an easy task. Proteins are chains of different types of amino acid units. To find out which amino acids are in the chain, and in what order, the protein must be cut, and the fragments isolated and identified. The difficulty is that there was no way of knowing where this cut had been made as all the bonds linking the amino acids together are identical. To solve this, Sanger invented a technique called the ‘N-terminal labelling technique’ which marks the start of the chain, which always contains a nitrogen group, with a detectable compound. The protein is then cut once and the resultant fragments separated; the fragment with the bound detectable compound is now known to be from the beginning of the sequence. This can be repeated and allows sequential identification of all the amino acids in the chain and their position.
After 10 years of hard work, Sanger obtained the full sequence of 51 amino acids that constitute insulin. This new method revolutionised the study of proteins and the Nobel Committee were swift to recognise the value of his work, awarding him his first Nobel Prize in 1958, just three years after his publication.
In the mid-1960s, Sanger launched himself into a new project: decrypting genomes–the whole DNA content of an organism. Francis Crick and James Watson had discovered the double-helix model of DNA and its complementary nucleotides in the early 1950s, but no direct link had been established between DNA and protein sequence. They had discovered the book of life but could not read it. Scientists knew that DNA, like proteins, had a chain- like structure. The challenge was to determine the order of adenine, thymine, guanine and cytosine–the chemical units from which DNA is made.
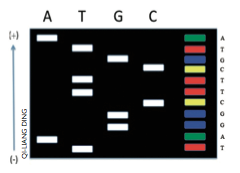
Sanger was already familiar with RNA sequencing, which he worked on during the late-1950s, and this gave him a head start. He combined established methods with novel ones and developed a ground-breaking way of sequencing DNA: the ‘dideoxy chain-termination’ reaction. In 1977, using this technique, he sequenced the first genome of a virus called phiX174.
His method’s elegance is in its simplicity. It uses
the natural process of DNA replication, which is stopped prematurely with a special terminator molecule. In the first round, a terminating adenosine is added to the reaction. As DNA gets copied, this terminator will eventually become incorporated at every ‘adenosine’ place of the DNA producing strands of varying lengths, all of which have an adenosine at the end. The process is repeated with terminating thymine, guanine and cytosine nucleotides. In the end, DNA strands of all lengths are produced, each terminating with a known A, G, C or T, spelling out the full sequence! In 1980, again only three years after the publication of the technique, Sanger was awarded his second Nobel Prize.
The ‘Sanger method’ has now been enhanced and automated using fluorescent nucleotides and laser scanners, and has unravelled the 3 billion base pairs of the human genome. However, it was Sanger’s seminal technique that paved the way to understanding the chemical basis of the genetic defects and the basis of inherited disease, some of which we can now screen by simply reading the patient’s DNA. Diseases, such as cancer, arise from mutations in the DNA. Modern DNA sequencing techniques tell us where these mutations are, and allow the development of more targeted drugs. By knowing the sequence of proteins we can now synthesise them ourselves, or transport the coding DNA into bacteria which then make the product for us. This has stemmed an explosion in therapeutic possibilities, such as hormone replacement therapy and the development of new antibiotics.
Beyond its vast medical implications, reading DNA is of great use to many other fields: palaeontologists have been able to confirm and map out more accurately the tree of evolution, criminologist use DNA evidence to confirm the presence of individuals in crime scenes and governments verify the contents of meat and foodstuffs, to name only a few examples.
At the age of 65, Sanger left his well-worn bench to retire to his family. He lived the last years of his life tucked away in the Cambridge Fens, practising carpentry, ‘messing about in boats’ and devoting himself to his family and garden. He outlived his wife, Margaret Joan Howe, after their 72 years of marriage. Although not a scientist herself, he described her as having “contributed more to [his] work than anyone else by providing a peaceful and happy home”.
Frederick Sanger impressed the scientific world with his novel techniques and disarming modesty. He referred to himself as “the chap who messed about in his lab” and declined the offer of a knighthood because he did not want to be called ‘Sir’. In 1993 he agreed for a research institute in Cambridge to be named after him, but only on the proviso that “it had better be good!”
Sanger’s techniques of protein and DNA sequencing propelled biology forward. His story illustrates how science often lingers, waiting for new technology to broaden its horizons. Indeed, exponential advances in science follow as often from the discovery of new experimental techniques as from the formulation of novel ideas. Just as the invention of the telescope opened up the universe to Galileo, and the microscope a new world of cells and bacteria, Sanger’s method offered us a new set of glasses: a pair that enabled us to begin understanding the genetic code.
Virginia Rutten is a 2nd year medical student at Trinity Hall