MONDAY, 3 MAY 2010
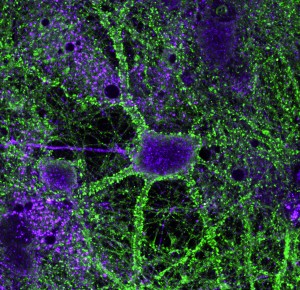
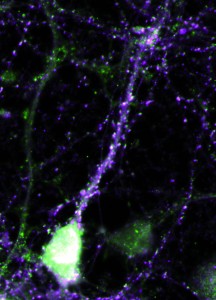
Tristan heintz’s picture of a hippocampal neuron is featured on this issue’s cover. The neuron is the green central cell, and is shown surrounded by red astrocytes and actin filaments in magenta. Tristan is a second year PhD student at the Cambridge Centre for Brain Repair and is studying brain plasticity and neuronal regeneration with the aim of restoring functionality to damaged neurons. Permanent neuronal damage is a common phenomenon that causes many devastating conditions including paralysis, Parkinson’s disease, stroke and multiple sclerosis. The brain we are born with is filled with neurons ready to make connections with one another in response to different environmental stimuli, a property known as plasticity. Up until the age of five, the brain develops rapidly and we learn most of the skills we need for life, so high levels of plasticity are vital. Later in life, plasticity reduces, but remains an active and essential process, and is believed to be key to how memories are made.Hippocampal neurons expressing either beta4 integrin in the cell body but not the axon (top) or alpha5 integrin in the dendritic spines (right).When damage occurs to part of the brain, plasticity is thought to be essential for the compensatory mechanisms that occur. Because of the greater plasticity in their brains, young children have a much greater ability to compensate than adults. For example, if a young child is blinded, the occipital lobes – normally responsible for interpreting signals from the retina – take over functions of nearby regions of the brain, for example those controlling touch as when reading Braille. This is known as cross-modal plasticity. Certain cases may result in superior skills involving touch or other modalities, which is why we sometimes hear of blind people having a ‘sixth sense’.
Most tissues, such as skin and bone, are very good at repairing themselves after injury, but the cells that make up the brain and spinal cord are not. The reason for this is believed to be two-fold. Firstly, after an axon is cut, it only mounts a meek effort at regeneration. Secondly, astrocytes, which are cells surrounding the neurons, secrete proteoglycans that stop the spread of degeneration from the initial site of injury. However, these molecules create an inhibitory environment that turns off plasticity and prevents natural repair. The result is a glial scar, much like a scar you would get after a deep cut where the skin is unable to repair itself properly.
Tristan is attempting to boost the axon’s repair mechanisms. His work centres around proteins called integrins, which span the cell membrane and allow neuronal cells to interact with the extracellular matrix surrounding them. Initially it was thought that the only function of integrins was to cling on to the matrix and ensure the cell remained in one place. However, it has been found that they have important roles in signalling, as they make direct contact between the interior and exterior of a cell.
The green staining in the image shows the location of alpha9integrin, which Tristan has overexpressed in hippocampal neurons in culture. By increasing the amount of alpha9integrin in the neuron, a damaged neuron gains the ability to regenerate itself. The integrins appear to do this by grabbing hold of the glycoproteins in the extracellular matrix and pulling broken ends back together, reforming the connection.
The extracellular matrix also plays a vital role in the decrease in plasticity as we age. Over time the matrix becomes very dense and full of inhibitory proteoglycans, which form a net around the axon. Research has shown that by breaking down this net with enzymes, such as chondroitinase, new connections are possible and rats are able to relearn simple tasks after brain injury.
By studying in detail the proteins and support structure surrounding neurons, it may be possible to kick-start the natural regenerative potential of our bodies and repair broken neural pathways in the central nervous system. This will then open up new treatment options to the many people affected by central nervous system damage.
Jessica Robinson is a PhD student in the Department of Oncology