TUESDAY, 21 MARCH 2017
MOSQUITO BORNE viruses like Malaria, Dengue, and Zika, account for more than half a million deaths per year. Vaccines have proved mostly ineffective against these killer diseases; and while prevention seems to be somewhat useful, the money and infrastructure needed to supply bed-nets and insecticides to their populations is simply insurmountable for developing countries in which these diseases are deadliest.However, what if all that was needed was the release of a few thousand modified mosquitoes? This is the solution that gene drives now offer, thanks to a helping hand from a new DNA cutting system, CRISPR Cas9. Unlike Crispr, gene drives are not new. In fact, they trace their origin as far as the 1970s to the popularisation of so called ‘selfish genes’.
"If scientists were able to insert a gene drive into mosquitoes, perhaps they could finally be eradicated"
The theory of evolution by natural selection proposes that genes that confer a ‘selective advantage’ i.e. help the organism survive and compete against its counterparts, are more likely to be passed onto that organism’s offspring than genes that don’t. But in 1976, Richard Dawkins, a prominent British evolutionary biologist, put forward the idea of ‘Selfish DNA’: DNA that spreads itself within a population’s genome while contributing nothing to the host’s likelihood of survival. Initially, Dawkins used this theory to explain the existence of the vast swathes of non-coding ‘junk’ DNA within the human genome. Over time however, it became apparent that coding DNA was also a type of ‘Selfish DNA’. Eventually, such coding DNA became known as gene drives.
These gene drives accomplish this selfish behaviour by breaking the normal rules of inheritance discovered long ago in the mid 19th century by the monk, Gregor Mendel. Typically, in sexual reproduction, a particular gene has a 50% chance of being inherited by a particular offspring. Gene drives manage to circumvent this cardinal doctrine of genetic inheritance, increasing the probability of their inheritance, so called ‘super-Mendelian inheritance’. The result of this property is that such drives can spread rapidly through a population. If scientists were able to insert a gene drive into mosquitoes, perhaps they could finally be eradicated.
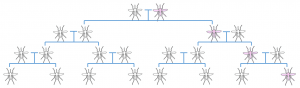 A Normal Mendelian inheritance; the altered gene (pink) has a 50% chance of being passed on to the offspring
A Normal Mendelian inheritance; the altered gene (pink) has a 50% chance of being passed on to the offspring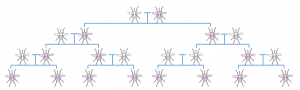 Super-Mendelian inheritance; a gene has an increased chance of being passed on to offspring, resulting in rapid spread of the gene through a population
Super-Mendelian inheritance; a gene has an increased chance of being passed on to offspring, resulting in rapid spread of the gene through a population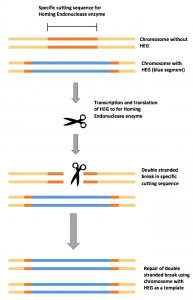 HEG drive mechanism
HEG drive mechanismIn 2012, the development of CRISPR Cas9 turned the bioscience community on its head. It presented a way of pasting any gene you wanted into the genome of an enormous range of organisms, by exploiting sophisticated bacterial self defence mechanisms. Fast-forwarding to 2015, two scientists at University of California, Ethan Bier and Valentino Gantz, had achieved what Burt had proposed in 2003. They had used this novel technology in a new method called mutagenic chain reaction (MCR), to turn a population of laboratory fruit flies yellow.
Following this remarkable development, the Gates Foundation poured $75million into research for gene drives to combat malaria, with a prediction that such technology would be ready for field use in Africa by 2016. So, what happened?
"The development of CRISPR Cas9 turned the bioscience community on its head"
With all technology involving genetic modification the ethical considerations of such actions must be reconciled, and the same is true for gene drives if not more so. In June 2016, a report from the National Academies of Science, Engineering and Medicine, in the USA concluded that, “There is insufficient evidence available at this time to support the release of gene-drive modified organisms into the environment.”. The report however urged scientists to continue research into gene drives and considered the idea of ‘highly controlled field trials’.
The fears surrounding gene drive technology are numerous, ranging from resistance to gene drives, to the transfer of malaria infectivity to other species of mosquito beyond the Anopheles variety, and even potential for biological terrorism.
Irrespective of the ethical purgatory in which gene drive technology is currently trapped, the resurrection of the idea (thanks to modern advances) proves that genetics research yields more than just promises for the future. However, with the Zika virus being downgraded by the WHO from a Public Health Emergency of International Concern in November 2016, perhaps all we need is education, bed-nets and insecticides after all.
Featured image: CDC Global
