MONDAY, 25 NOVEMBER 2013
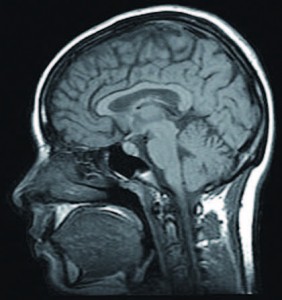
Used for centuries in religious ceremonies, psychedelic drugs like magic mushrooms have a long and vibrant history, and could one day be used in the clinic to treat depression and anxiety. Whilst the 40s and 50s saw extensive research into the mind-altering effects of psychedelics as potential psychiatric aids, promising research ground to a halt after drug misuse and media scares led to psychedelics being made illegal. Following a 50-year research hiatus, scientists are re-discovering the potential of drugs like magic mushrooms, ecstasy and ketamine as clinical aids in psychiatry. In the last decade, renewed interest has led to pre-clinical studies conducted on a whole host of drugs including lysergic acid diethylamide (LSD), psilocybin, ibogaine and 3,4-methylenedioxy-N-methylamphetamine (MDMA). Still, relatively little is known about how psychedelics act in the brain to produce such profound changes in consciousness.Amongst those interested in the potential medical benefits of certain under-researched illegal drugs, David Nutt and his colleagues of Imperial College London are interested in using psilocybin, the active compound found in magic mushrooms, as a potential treatment for depression. Psilocybin has already shown promising results in the treatment of anxiety, pain, and existential crises associated with end-stage cancer, and as a potential new treatment for obsessive-compulsive disorder. Using functional magnetic resonance imaging (fMRI), Nutt and colleagues have provided the most detailed account to date of how the psychedelic state is produced in the brain.
Specifically, 30 volunteers were scanned using fMRI during intravenous infusion of placebo or psilocybin. Psilocybin produced profound changes in consciousness and was found to switch off activity in two brain areas in particular: the anterior cingulate cortex and the medial prefrontal cortex. These form part of the brain’s ‘default mode network’, a system that is active when individuals are thinking about themselves, the future or recalling memories, and is also overactive in depression. Interestingly, the greater the decrease in ‘default mode network’ activity by psilocybin, the greater the intensity of subjective drug effects. These are the first results that help to explain how psilocybin could be useful in treating depression.
These findings prompted Nutt and colleagues to conduct a second study, in which they investigated whether psilocybin could facilitate access to personal memories and emotions. The group again used fMRI to scan 10 volunteers, receiving either psilocybin or placebo, while they viewed autobiographical memory cues. Whilst both placebo and psilocybin enabled the recollection of memories in response to the cues, psilocybin produced especially vivid ones and sensory areas were uniquely activated by memory cues under the drug. Moreover, greater vividness was associated with improved subjective well-being at follow-up two weeks later. It appears that by switching off an over-active default mode network, psilocybin enables the senses to run free and could lead to longer-lasting positive effects on mood. Researchers hope to harness these powerful mind-altering effects of psilocybin to reverse pessimistic thoughts associated with depression.
Another drug with promise in the clinic is MDMA, the active component in the street drug Ecstasy. MDMA increases empathy and encourages recall and exploration of painful memories, rendering it particularly suitable for assisting psychotherapy. Several pilot studies are revealing beneficial effects of MDMA for the treatment of post-traumatic stress disorder (PTSD). In 2011, the first controlled trial of MDMA-assisted psychotherapy on treatment- resistant PTSD patients showed that it produced significant improvements in symptoms compared with psychotherapy alone. Furthermore, the long- term follow-up of the participants found that the MDMA effects were maintained for over 3 years on average. These promising studies suggest psychedelics may offer hope to patients who do not respond to conventional treatments and therefore highlight their potential therapeutic value.
Despite these findings, research with psychedelic compounds remains controversial; MDMA, like psilocybin and other psychedelics, is illegal and governmental regulations continue to hinder research into its effects. Coloured by the drug culture of the 60s and extensive media coverage of MDMA misuse, psychedelics continue to have a negative image in society. The legal scheduling of psychedelic drugs makes it difficult for scientists to study them, and pharmaceutical companies are often unwilling to undergo the long and costly process of obtaining a license to manufacture the drugs. But, even if they were shown to be beneficial, current regulations would make the use of these drugs difficult. These laws were set out to protect people from the harm that psychedelic drugs can cause when used recreationally, thus rendering the fear of addictive abuse potential a legitimate cause for concern. However, it is becoming apparent that these drugs could be of immense benefit to patients who fail to respond to conventional treatments, if used in a carefully controlled clinical setting.
A shift in the way society perceives these drugs could be advantageous to patients and recreational users alike, by facilitating development of new treatments and even recreational drugs. For instance, we have witnessed major advances in the understanding and treatment of drug addiction, a major worldwide health problem, for which there are currently limited treatment options. Novel approaches, which treat drug users as patients rather than criminals, include anti-drug vaccines for cigarette smokers or cocaine addicts. The anti- drug vaccines generate antibodies that bind to the drug and prevent it from reaching the brain; anti-cocaine vaccines are already available to treat cocaine overdose. It is possible to imagine a future in which vulnerable people would be vaccinated to protect them from developing an addiction in the first place. This would pave the way for bringing psychedelics back into the clinic, as researchers believe that psychedelics could help recovery from addiction to drugs such as opiates, alcohol and nicotine. In addition, increasing acceptance of psychedelic research may allow for the development of better synthetic alternatives.
Paradoxically, thinking of a safer drug future might even one day extend to those drugs used recreationally, with the development of less- harmful and improved drugs than the ones we have today. Nicotine substitutes, including the nicotine gum, patches and lozenges, are already available on the market to help combat cigarette smoking. David Nutt and colleagues have even proposed a drug related to Valium as a more innocuous alcohol substitute. This novel ‘harm-free’ alcohol has an antidote, which would allow revelers to sober up instantly at the end of a party and drive home quite safely.
These exciting developments are only an example of what our future drug landscape might look like. The next 20 years could witness a plethora of potentially safer recreational drugs and medicines for mental health. However, this may only happen with a change in social attitudes and drug policies. For instance, while the impact of addiction on society is significant, the social stigma associated with addiction and use of recreational drugs means that pharmaceutical companies have little incentive to develop treatments for addicts to illicit substances. Similarly, prohibitive scheduling of psychedelics may be preventing medical advances that could help relieve patients suffering from depression, anxiety and addiction. A better understanding of how the human brain works has led to changes in the way we treat drug addiction. These advances illustrate the importance of framing new treatments in a modern context. Perhaps with psychedelics too we need to separate their therapeutic use from their historical recreational misuse and open up new possibilities for the treatment of severely debilitating illnesses.
Camilla d’Angelo is a 2nd year PhD student in Experimental Psychology