WEDNESDAY, 14 MAY 2014
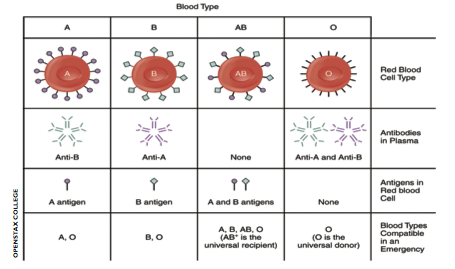
Red blood cells are coated in a set of carbohydrate molecules. Before the turn of the 20th century, these seemingly innocent carbohydrates were responsible for many deaths following blood transfusions. This is because the carbohydrates on red blood cells characterise the blood groups, and blood group matching is essential for safe blood transfusions. But what are blood groups and how are they associated with infections?In 1900 Dr Karl Landsteiner discovered the different blood groups in humans-A, B and O. Dr Landsteiner showed that mixing blood from an A-positive and a B-positive person caused disastrous clumping or ‘agglutination’ of the blood in a test tube. This discovery finally clarified why some blood transfusions were fatal.
Subsequent work elucidated the mechanisms behind these dramatic reactions. All red blood cells are O-positive by default and it is the presence of a specific enzyme, either an A or B enzyme, that dictates whether an A or B sugar will be added to the core carbohydrates that coat red blood cells.
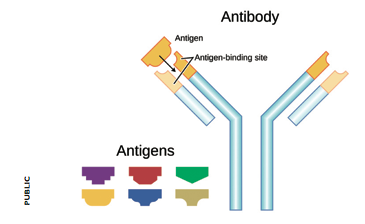
Agglutination of red blood cells occurs when antibodies (immune proteins that recognise and help destroy harmful molecules) target foreign blood group carbohydrates. Antibodies against foreign blood groups naturally develop in humans. However, the immune system of an A-positive individual will not allow the production of antibodies that target A sugars. In other words it will not recognise its own cells as foreign or threatening. Similarly, in a B-positive individual no anti-B antibodies develop. If someone’s blood group is O, their immune system will make both anti-A and anti-B antibodies. If someone with an O-positive blood type is transfused with A- or B-positive blood, their antibodies will destroy these new red blood cells. In 1 out of 20 cases this leads to sudden death.
But why do the different blood groups exist? Is there a benefit in having different blood types?
Exactly why there is a mixture of different blood types in many species is indeed a mystery. One hypothesis is that pathogens may have played a role in the evolution of blood types. Many pathogens use carbohydrates from the cell surface as receptors to bind to the cell. Receptors can also be used as anchors to prevent from being swept away and they might help a pathogen enter the cell or release its toxins inside it. Depending on their blood type, people are more or less susceptible to certain pathogens. If you do not express a pathogen’s receptor but your neighbour does, you will be safe from infection but your neighbour might fall ill.
Cholera was the first infectious disease shown to affect people in different ways according to their blood group. The responsible bacterium, Vibrio cholerae, causes severe watery diarrhoea and can be fatal if appropriate treatment is not received. Outbreaks in the developing world usually have devastating consequences, as happened in a recent epidemic in Haiti, which resulted in over 8,000 deaths.
In the 1970s researchers noticed that individuals with blood group O were more likely to become infected with cholera than people with blood group A or B. Because V. cholerae is a bacterium that causes infection in the gut, the fact that blood groups mattered was a mystery.
It turned out that A, B and O antigens are not only expressed on red blood cells. They are also found on epithelial cells, including those lining the gut. A more accurate term for these carbohydrates therefore includes the prefix ‘histo’, meaning tissue. These antigens are found in the gastrointestinal tract of many different animal species, but only humans and great apes express the histo-blood group antigens on the surface of their red blood cells.
Once the properties of histo-blood group antigens were understood in more detail, an association between cholera infection and blood groups could be explained by focusing on the carbohydrates present in the gut. The current theory is that A and B carbohydrates on the cell surface block the binding of the cholera toxin to its receptor molecule, ganglioside GM1, which is also located on the cell surface. People who do not have A or B carbohydrates on their cells cannot disrupt this interaction, and hence the cholera toxin enters the cell and causes disease.
People with blood group O are at an evolutionary disadvantage in a cholera-endemic region. This may explain why the proportion of O-positive people in such areas is significantly smaller than in parts of the world where cholera infection is very rare. If a person dies from a cholera infection, they cannot pass on the O blood group to their offspring and hence the proportion of O individuals decreases. This suggests that the blood group distribution in host populations can be substantially altered by pathogenic threats.
For a long time, researchers had suspected that the carbohydrates that determine blood groups could enable pathogens to enter cells, not merely their toxins. In 2002, the first pathogen that uses receptors in this way was finally identified. Human norovirus, the cause of the winter vomiting bug that infects up to three million people in the UK every year, was shown to bind to blood group carbohydrates and enter the cell. The first norovirus strain studied turned out to bound strongly to A and O blood groups, but less efficiently to B. This led to the hypothesis that people with B blood group would be less susceptible to the disease, a hypothesis that was confirmed shortly afterwards.
Many other strains of noroviruses have now been studied, and all of them can recognize at least one of the A, B and O blood group antigens.
Human red blood cells are coated by carbohydrate molecules which determine the blood type. Interestingly, the strains that have emerged more recently are able to bind to the three bloodgroups. The most prevalent norovirus strain in the world today is known as GII.4, which can recognize virtually any blood type. This suggests that pathogens can evolve to encompassing a broader host number.
As well as V. cholerae and noroviruses, additional pathogens are now regularly being identified that interact differently according to their hosts’ histo-blood group. These include Escherichia coli, rotaviruses and the dengue virus. Each pathogen attacks blood groups in slightly different ways, which means that in a world full of different infectious diseases, eradication of a single blood group is very unlikely to happen. Unless we find a way to conquer all the blood group-associating pathogens, we will have to keep checking blood types thoroughly prior to blood transfusions for millennia to come.
Sarah Caddy is a veterinary surgeon and a 3rd year PhD student at the Division of Virology