SATURDAY, 29 JANUARY 2011
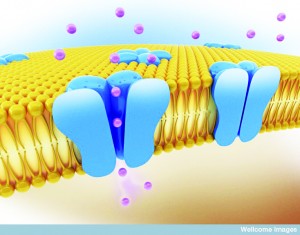
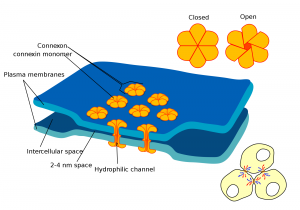
Whether communicating sensations of pain or the need for insulin release from the pancreas, cell to cell communication is vital for our survival. Just as we communicate our desires, emotions and situations through a variety of modes such as speech, facial expression and gesticulations, cells have evolved a multitude of ways to communicate with each other. Of these methods, juxtacrine signalling—communication between adjacent cells — is the most effective means of cellular signalling in fundamental processes in the body such as the beating of our hearts. In addition, it has formed the basis of a novel means of cancer treatment and has been implicated in embryological development.Juxtacrine cell signalling, unlike other signalling methods, requires the communicating cells to be in physical contact with each other. These regions of physical contact between the cell membranes are known as gap junctions. They allow cells to communicate their intracellular condition to adjacent cells by the presence of membrane-spanning channels which connect the cells’ interiors. This allows the regulated exchange of intracellular peptides, ions and other chemical components that are present in the internal environment of the cell. In this way, an external signal affecting the internal environment of a single cell can be transmitted across tissue. One of the best examples of the body utilising this method of signalling is in cardiac tissue. Special ‘pacemaker’ cells in the heart contain channels allowing the constant leak of positively-charged sodium ions into the cell. This increases the charge inside the cell and, beyond a certain threshold, this elicits the generation of an electrical signal known as an action potential.
Action potentials travel within the cell cytoplasm as waves of positive ions. Since cardiac muscle cells are connected to each other via gap junctions, these waves of positive ions can cross cell membranes and are transmitted across all the cardiac muscle cells. This increased positive charge within the cardiac muscle cells causes them to contract. The result: a synchronised heartbeat. The conduction of these action potentials throughout the cardiac muscle tissue can be monitored in an electrocardiogram (ECG) which is a common test performed on patients complaining of chest pains, palpitations and respiratory distress. An ECG test can help cardiologists detect irregular heart rhythms caused by faulty conductance of electrical signals through the heart, and even identify patients at risk of a heart attack.
As well as allowing synchronisation of cellular reactions, juxtacrine signalling also provides speed in signalling. Since gap junction-mediated signalling relies on electrical signal transmission via the flow of ions, the speed of signal transduction far exceeds other means of signalling which rely on bulk flow through the circulation or passive diffusion through intercellular space.
In addition, electrical signal transmission via gap junctions rules out the possibility of chemical fatigue, a problem that is faced in nerve to nerve transmission via chemicals. Nerves rely on neurotransmitters which need to be manufactured from chemical subcomponents. As a result, repeated stimulation of nerves can cause the rate of release of neurotransmitters to exceed the rate of their production. The weakening of signals transmitted due to insufficient neurotransmitters is known as chemical fatigue. With gap junctions, ions are constantly transported across all cell membranes via ion channels, such as the sodium pump, and any ionic gradients naturally dissipate. This ensures that any localised depletion of ions is rapidly replenished and baseline conditions are quickly restored, avoiding any form of fatigue.
The characteristics of speed and lack of fatigue could have given the transmembrane connexin proteins, which form the gap junction channels, an evolutionary selection advantage. As described by Dr Robert Malenka, in his book on intercellular communication, many species of fish, amphibians and crustaceans use gap junction—mediated cell signalling to initiate what is known as the escape response.
In crustaceans, such as crayfish and lobsters, the escape response involves a ‘tail flip’ which requires the coordinated movement of the segments of the tail. Each segment receives signals from a single neuron and these neurons are coupled together via gap junctions. Thus, the neurons effectively function as a single unit that enables the quick transmission of electrical impulses through the tail. The speed of signal transmission provided by gap junctions thus enables a rapid ‘tail flip’ response that is crucial in escaping from predators and other dangers.
While gap junctions can be said to be indispensable, the full extent of their roles in cell signalling is still being unravelled. Recent research has implicated gap junctions in embryogenesis and early cell differentiation. Findings suggest that gap junctions may provide cells within the embryo with a quick and effective means of communication before the circulatory system has been established. Crucial left—right patterning in embryos that directs the development of the heart in the left side of the chest may also be communicated to cells via juxtacrine signalling.
Recently, scientists have started to investigate the use of gap junctions as part of a novel tumour treatment strategy. During typical melanoma progression, the production of the ubiquitous transmembrane connexin 43—a protein involved in the formation of gap junctions—has been observed to drop significantly. Infection of mouse melanoma cells with diarrhoea-inducing Salmonella typhimurium caused production of the connexin 43 protein to be upregulated, allowing these melanoma cells to form gap junctions with dendritic cells of the body’s immune system.
The research team found that these gap junctions allowed melanoma-specific peptides to enter the dendritic cells. The experiments showed that the dendritic cells exposed these peptides by transferring them to their cell surface in a process known as cross-presentation. This allowed recognition of the melanoma peptides by helper T-cells of the adaptive immune system, which can activate pathways to destroy cells with those specific melanoma peptides. The team hopes to devise a vaccination scheme in the coming months. The vaccination scheme will involve withdrawing melanoma cells, dendritic cells and T-cells from individual patients. By first infecting the melanoma cells with Salmonella typhimurium to reinduce connexin 43 production, and then placing these cells together with the patient’s immune cells, the patient’s T-cells will be ‘trained’ to identify the peptides specific to the patient’s melanoma. These patient-melanoma-peptide-specific T-cells will then be reinjected back into the individual patients to initiate an immune response against the melanoma cells.
Like many other novel therapies, this type of cancer therapy focuses on individual patient characteristics. By using the patient’s own melanoma cells and immune cells to induce gap junction mediated recruitment of the immune system, the team hopes to have found a potent method of targeting and destroying cancer cells. “We are inducing an immune response to that ‘fingerprint’ which is specific for the tumour” said Dr Maria Rescigno, an immunologist at the European Institute of Oncology in Milan who was involved in the study. If approved, clinical vaccination trials will start by June 2011.
The role of gap junctions in immune responses has only recently come to light, with research revealing that gap junctions are heavily involved in cross-presentation. This suggests the frequent involvement of juxtacrine signalling in the protection of the body against viruses, bacteria and even primordial tumours. As such, further research into gap junction—mediated recruitment of the immune system seems to promise new options for individualised treatment strategies as well as mass-spectrum therapies against viruses like influenza and the common cold.
Rhea Chatterjea is a 2nd year undergraduate in the Department of Medicine.