THURSDAY, 15 MAY 2014
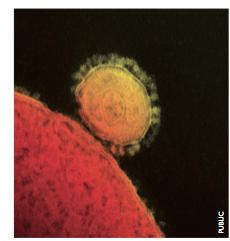
Our usual battles with viruses are mundane. After catching a cold we rest at home, tissues in hand, and wait for our immune system to squash the unwelcome bug. But what about other viruses out there? Dangerous strains of influenza, say, or perhaps an unknown enemy altogether? For unknown pathogens, worldwide surveillance for clusters of human or animal disease provides a way to sound the alarm before the bugs go viral.In June 2012, Dr Ali Mohamed Zaki of the Virology Laboratory in Dr Soliman Fakeeh Hospital in Jeddah, Saudi Arabia, assessed a 60 year-old male patient admitted with pneumonia and acute renal failure. Taking a sputum sample from the patient, who would later go on to die of his infection, Dr Zaki was unable to identify the infectious agent with routine lab tests. He sent a sample to Dr Ron Fouchier in the Netherlands for further analysis. The diagnosis: a previously unidentified coronavirus.
We saw such an event in recent history. In 2003, the SARS coronavirus (SARS-CoV) hit headlines, sent face-mask sales soaring, vilified global air travel, infected roughly 8000 people and resulted in a case fatality rate of over 9 per cent. In the over 50s, nearly 50 per cent of infected people died. Now a new coronavirus had been uncovered– again the cause of a lethal pneumonia.
Further cases of the newly named Middle East respiratory syndrome coronavirus (MERS-CoV) occurred in Jordan, Saudi Arabia, Qatar and the United Arab Emirates (UAE), with infected air travelers carrying it further to Tunisia, Italy, France and the UK. With time it became no less devastating. As of early 2014, the total number of laboratory-confirmed cases was 184, as assessed by the World Health Organisation (WHO), with 80 being fatal.
To gain the potential to become pandemic–a global outbreak of the virus and its disease–MERS-CoV would require the ability to spread easily from person to person. Like related coronaviruses, transmission was assumed to occur efficiently through the air. But whilst early case reports describing MERS-CoV outbreaks within family groups and healthcare workers made for grim reading, it transpired that sustained close contact between individuals was necessary for the virus to spread.
Despite isolating infected individuals to limit the spread, recurring isolated outbreaks continued. With the world watching, scientists and medical professionals raced to identify the source of the new respiratory disease. With the majority of confirmed cases, the search focused upon the Kingdom of Saudi Arabia.
Where were people contracting the virus? To persist in the human population, continuous spread between people is a necessity. Was the severe disease the tip of a widespread ‘MERS-iceberg’? Probably not–studies from 2013-14 found little evidence of MERS-CoV circulation in humans in either Jeddah or the Eastern Region within Saudi Arabia. So where was the virus coming from? And why was the outbreak limited to the Arabian Peninsula?
A clue came from the original virus isolated by Dr Zaki. Analysis of its genome determined that two bat coronaviruses, HKU4 and HKU5, were its closest relatives. SARS had also originated in bats infecting palm civets later sold in live animal markets in China, where human transmission could occur. Thoughts turned to the existence of an animal reservoir; an animal population in which the virus naturally circulated but from which it was now transmitting to humans. To date, research groups have indeed found highly related coronaviruses in Saudi Arabian and South African bats, with short stretches of MERS- CoV RNA detected in an individual bat near the site of the first human infection. Virologically speaking, bats are veritable zoos, and coronavirus infections
are common. A 2013 mBio study determined that 58 different species from nine diverse virus families exist within just one bat species (Pteropus giganteus; the Indian flying fox). However, with bat-human contact rare, circumstantial evidence suggested another, unlikely, animal responsible for human infection: the dromedary camel.
When using the phrase ‘animal reservoir’, most imagine the animal kingdom spreading parasites and viruses via malarial mosquitoes, rabies-infected dogs, and Dustin Hoffman chasing a screeching ebolavirus- riddled capuchin monkey in the 1995 film, Outbreak. Not camels.
A 2013 report published in Lancet Infectious Disease suggested otherwise. When humans are infected with a virus, the immune system swings into action to clear the contagion. It also holds a grudge. Anti-viral cells and immune proteins called antibodies linger in our bloodstream, ready to pounce should a relative of the virus try to infect us again. The camel immune system is no different. Looking for these telltale signs of infection in retired racing camels from Oman and the Canary islands, researchers of the 2013 study found antibodies that bound to the MERS-CoV spike protein in 64 of the 155 animals tested, including all 50 from Oman. Furthermore, the antibodies did not react with a bovine coronavirus known to infect camels. Whilst no infectious virus or viral RNAs were isolated, making it impossible to determine whether they currently carried the virus, the camels had clearly been infected with a MERS-CoV-like virus.
Further evidence arose from the 11th known MERS-CoV fatality. A 43 year-old man was rushed to intensive care with pneumonia after spending days attending to a sick racing camel. Crucially, the Saudi Ministry of Health detected the presence of MERS- CoV RNA within the animal, confirming the presence of a MERS-CoV-like virus at the time of illness. The camel recovered. The owner did not.
However, despite evidence of camel and bat infection, there is no clear model for the transmission of MERS-CoV from infected animal hosts to humans. For many patients, animal contact was either not found or could not be determined. Is it possible that the trade in camel meat products in Saudi Arabia and the UAE may spread the virus through the food supply? It’s a weighty claim, and whilst the WHO takes a cautious approach, stating that “sick animals should never be slaughtered for consumption”, there isn’t direct evidence to suggest this is a source of transmission. A 2013 report of simultaneous illness in goats and their MERS-CoV- positive Qatari caretaker may yet implicate more animals into the virus transmission cycle, but the fact is we still do not know how the majority of people become infected.
So, whilst continued surveillance and virus research are necessary to determine exactly how humans are contracting the virus, a final question begs answering: why are we only seeing human MERS-CoV infections now? Intriguingly, camels may have had the virus for over a decade. A report in Emerging Infectious Diseases posted online in January 2014 has shown that frozen camel serum samples from 2003 also test positive for MERS-CoV antibodies–nine years before human infections were first recorded. Have human infections occurred before but gone unnoticed–simply recorded as pneumonia without a second thought? Or has the virus mutated in its natural host (or hosts) and gained the ability to transmit to humans for the first time? And, if so, will human infections become ever more common? Unfortunately, as with much of MERS-CoV’s mysteries, we are going to have to wait and remain vigilant to find out.
In the meantime, keep a suspicious eye on the camels, and watch out for their spit.
Michael Nicoll is a post-doctoral researcher at the Department of Pathology