MONDAY, 9 MAY 2011
Schizophrenia is a chronic, debilitating, psychotic mental disorder that affects about 30 million people worldwide and is characterized by a range of symptoms, both negative and positive . Current diagnosis methods are subjective as they are based on psychiatric interviews. The biochemistry underlying this disorder, however, is still mostly unknown. Schizophrenia is likely to be a consequence of serial alterations of a number of genes and proteins that, together with environmental factors, will lead to the establishment of the disorder.Gaps in our understanding of schizophrenia could be effectively addressed through proteomic approaches. Proteomics is a relatively young science which arose from the concept of a ‘proteome’, defined as “the study of the total set of proteins expressed by a cell, tissue or organism at a given time under defined conditions.”
In a partnership with BrainNet Europe [1], post-mortem brain tissue has been collected from schizophrenia patients and demographically matched healthy control individuals, for the purpose of analysing their proteomes. brain proteins were , using a shotgun mass spectrometry approach. Which allows rapid identification of all proteins in a sample by combining chromatography and spectrometry techniques.
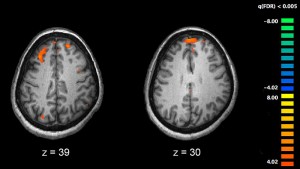
The proteome analysis of brain samples of schizophrenia patients shows us that the most prominent alterations in protein expression are related to energy metabolism, followed by oligodendrocyte and cytoskeleton dysfunction, affecting cell signalling and organisation.
In schizophrenia brain tissue, glycolysis was the most affected pathway. This is the starting point for respiration, the process by which cells generate usable energy. In glycolysis, glucose is processed through the many enzymatic reactions leading to two molecules of pyruvate which can be converted for use by the Krebs cycle, in mitochondria. These findings highlight the role of impaired glucose metabolism in schizophrenia, supporting previous results such as hyperglycemia, impaired glucose tolerance and/or insulin resistance in patients.
Brain cells are highly active and as such contain a relatively high number of mitochondria, responsible for fulfilling 95% of the cell’s energy requirements. Alterations in mitochondrial energy conversion, caused either by internal or external factors, may explain the impairment of energy production observed in schizophrenia. Altered mitochondrial function is also likely to increase production of reactive oxygen species which may lead to DNA damage, altering gene function and ultimately protein expression. These processes could be responsible for impaired neuronal plasticity and neurotransmission, leading to characteristic schizophrenic behaviour.
Oligodendrocytes are a type of cell which support correct brain cell function and play several roles in the central nervous system (CNS), including signaling, growth factor production, . However, the most important role of oligodendrocytes is to insulate the axons in the central nervous system (CNS), providing an electrically-insulating layer, made of myelin, that facilitates signal transmission through nerves. Disruption of the myelin sheath leads to ion leakage, resulting in reduced nerve activity , and compromised brain function. Brain imaging, transcriptomic and epigenetic studies have clearly demonstrated the dysfunction of oligodendrocytes in schizophrenia.
Some of the differentially expressed proteins found in this study, such as myelin basic protein (MBP) and myelin oligodendrocyte glycoprotein (MOG), were altered in the cerebrospinal fluid of schizophrenia patients compared to healthy individuals. Our findings support the idea that some kind of neurodegenerative process might be occurring in schizophrenia.
The cytoskeleton is a series of fibres inside cells which control the shape of cells and the location of different structures within cells. Cytoskeletal proteins have tissue-specific patterns of expression that, if altered, can influence cell shape, polarity, neuritogenesis (outgrowth), and, neurotransmission by nerve cells. Some proteins such as tubulin beta (TUBB), glial fibrillary acidic protein (GFAP), neurofilaments M and L (NEFM and NEFL) and dynamin 1 (DNM1) are differentially expressed in schizophrenia brain tissue. Changes in proteins affecting neuronal development, signalling, nucleic acid (which makes DNA and RNA) processing, cell division and cell death have also been recorded.
When we started our experiments on the proteome of schizophrenia brain tissue, the main aim of global protein expression studies in schizophrenia brain tissue was to reveal differentially expressed genes or proteins that could yield potential markers for use in diagnosis of schizophrenia. It become very clear, from this research, that such a complex disorder as schizophrenia, which involves the interaction of multiple genetic and environmental factors, wouldn’t be identifiable using a single biomarker, but instead requires a set of them.
The good news is that proteomic and metabolic analysis of schizophrenia samples yielded a diagnostic test with approximately 85% diagnostic accuracy. This test, which was developed in the University of Cambridge, is a promising improvement for a schizophrenia patients worldwide [2], [3].
In addition, we have gained a more comprehensive knowledge of the mechanisms which cause schizophrenia which may contribute towards establishing more targeted treatments for this debilitating and isolating disease.
Written by Daniel Martins-de-Souza