MONDAY, 10 SEPTEMBER 2018
In this age of modern medicine it is easy to forget that only a century ago, an infected scratch from a bramble could have cost you your life. Alexander Fleming’s discovery of the first antibiotic – penicillin – opened up a wealth of opportunities. Now an infected cut is just a mild inconvenience, and surgeons can perform revolutionary operations, safe in the knowledge that a bacterial infection will not destroy their work (or their patient’s life!).But doctors are becoming uneasy. As the usage and diversity of antibiotics has increased, so has the incidence of resistant bacteria that still replicate under chemical onslaught. With hindsight, it is easy to realise that antibiotics would provide a strong selective pressure on microbes. Bacteria replicate rapidly by each dividing into two identical cells. Every new generation yields ‘mutant’ cells where the genetic material has not been correctly copied. Occasionally a mutant arises that can defend itself from the antibiotic, so escaping the drug- induced massacre. Unfortunately, it only takes one such mutant to resume an infection. With exposure to every new antibiotic, bacteria have another chance to evolve. And yet, while the Western world fights never-ending bacterial battles with a chemical arsenal, a treatment continues in the East – bacteriophage therapy.
Bacteriophages are viruses that attack and replicate in bacterial cells; replication of the viruses eventually leads to membrane rupture and death for the bacterium. Remarkably, despite their minuscule size, they were discovered in the early 1900s by Félix d’Herelle. Working in his Parisian laboratory, he observed the culling of dysentery bacteria in faeces from a patient. As was the nature of science in the pre-war era, D’Herelle trialled his phage ‘soup’ first on himself, as a test of safety, and then on dysentery sufferers, effecting a cure within only 24 hours. D’Herelle admitted that this small sample size could not provide definitive evidence of the benefit of phage therapy, but the discovery was promising and had the potential to be revolutionary.
So why are we not using phages in treating bacterial infections? Actually, phage ‘cocktails’ are used as a first line treatment in several Eastern-European countries including Russia, Georgia and Poland. Phage therapy is predominantly absent in Western countries, with a clear preference for antibiotics. A disordered history of political unrest and poor experimental technique may be to blame.
Initially the chaos of the First World War diverted D’Herelle’s time from research to vaccine production and although he continued to run experiments during the night, this significantly slowed developments. Following the war, interest in phage grew and researchers who had caught onto the hot topic began myriads of studies. Many of these experiments were poorly controlled, and dosages of phage were not even reported. Patients could have been receiving ineffective doses, which could explain negative results. It was even proposed that the bacteriophages were not the supposed assassins, and instead an antibacterial compound or protein in the phage mix was responsible. This was still a viable hypothesis up until the mid-1930s, when the phage broth was heat-treated to destroy the viruses and injected into sick mice – these mice died.
Western countries showed great interest in bacteriophage, with the US hopeful that the treatment would prove effective. During the first few years of the Second World War, American scientists carried out careful research into the behaviour of the virus that preyed upon the group of bacteria, Shigella – the common cause of dysentery. By 1945, the US teetered on the edge of clinical trials but antibiotics won the race. American scientists rediscovered Fleming’s work and penicillin was hailed as the new ‘wonder-drug.’
While Western countries remain enchanted by antibiotics, Eastern-European countries continued with the antiquated phage. The war may have driven the US towards the quick fix provided by antibiotics, but it also led to the establishment of the Iron Curtain, limiting Eastern access to antibiotics and forcing investment in phage research.
The Eliava Institute in the country of Georgia was established in the 1920s, and – despite suffering the terror of Stalin’s reign – it is now viewed as a leading centre for bacteriophage research. It not only focuses on phage discovery and characterisation, but routinely treats patients. Mass production of phages has been attempted using large scale bacterial cultures, and innovative bandages impregnated with phage have shown efficacy in treatments. A rise in resistant bacteria has led to people travelling to Georgia to receive treatment, or doctors posting swab samples in an attempt to find a cure for their patient. Where antibiotic options fail, bacteriophages are still able to defeat superbugs.
So why are phages able to do what antibiotics cannot? Viruses are under pressure to infect a cell. Bacteria provide a nutrient-rich home for phage replication and without a host a virus is doomed. Similar to bacteria, phages reproduce rapidly and acquire random mutations. Even as the bacteria develop mechanisms to counteract infection, the phages look for a new way to come out on top.
Unfortunately, this constantly changing medicine often strikes fear into the pharmaceutical companies and health institutes. While an antibiotic will remain in its static form, a mixture of phages used in one week, or even one day, may become very different the next. Georgian scientists even refresh these mixtures, adding new strains of phage to maintain their potency. The resulting ingredients may be somewhat unknown. And this is only touching upon some of the anxiety around phage therapy. As humans, we are naturally afraid of viruses. Pathogens such as polio, HIV and rabies, have been responsible for thousands of deaths worldwide. You might worry we are about to create the next pandemic virus, but in fact the very name ‘bacteriophage’ hides one of their greatest benefits: specificity to bacteria.
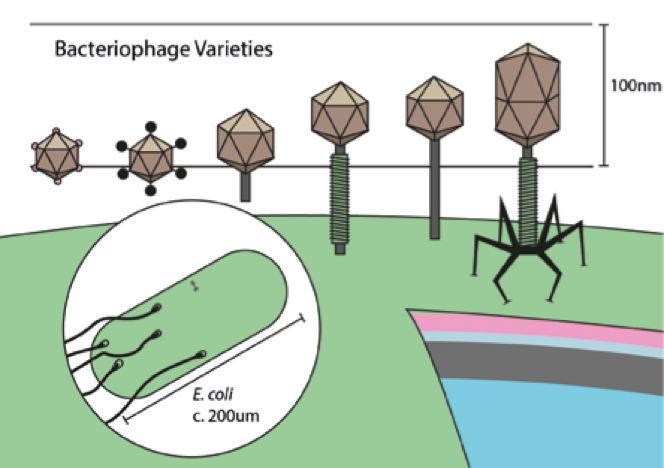 The size and shape of the capsid, the ‘head’ of a bacterio- phage, varies with the size of its genome, which can contain up to hundreds of genes
The size and shape of the capsid, the ‘head’ of a bacterio- phage, varies with the size of its genome, which can contain up to hundreds of genesThis is why current, high quality research is so important. Recently there has been renewed interest in bacteriophage research, with a number of clinical trials taking place. A large scale collaboration known as ‘Phagoburn’ even involved several European countries including France, Switzerland and Belgium. Many of these studies are presently focussed on the usage of phage ‘cocktails’ to treat infected burns, commonly caused by Pseudomonas aeruginosa. This bacterium is also the nemesis of cystic fibrosis patients and leads to dangerous respiratory infections. Results of experiments in a mouse model of cystic fibrosis were published only this year. Delivery of phage mixtures into infected mice resulted in either complete clearance of the bacteria within a few days, or reduced bacterial counts by around 70% even after a week of infection.
But not all studies have produced such good results: attempts to treat some infections, such as the Staphylococcal infection mastitis, have been futile due to the bacteriophages themselves being rapidly eliminated from the mammary gland. Studying efficacy of phage treatment on mastitis suggested that certain tissues may mount immune responses against phage, with milk from the study animals containing large numbers of inflammatory cells. An immune response not only damages healthy cells, but may inactivate the phages through neutralising antibodies. It is possible that phages may need to be administered differently for distinct infections.
Phages may not become the new ‘wonder drug,’ but many believe that they would be a useful last resort for patients whose superbugs no longer respond to antibiotics. Ultimately we must decide how much time should be spent on research and clinical trials before we transfer phages to the hospital bed. Antibiotic discoveries are slow and there is fear of a return to a time when bacteria could not be controlled. Viruses may play a key role in preventing this possibility, but only time will tell if our medicinal infrastructure, and our culture, can accept them.
Laura Upstone is a 3rd year in Biological Natural Sciences at Pembroke college. Artwork by Oran Maguire
