FRIDAY, 7 JULY 2017
In the news, in conversation, in the cafe - you have probably heard of epigenetics. But what exactly is it?
Epigenetics literally means ‘on top of genetics’. Whereas genetics is the study of genes, which are stretches of DNA containing the information needed to make proteins, epigenetics is the study of how the cell uses those genes to make proteins.
 Susanna McLaren
Susanna McLarenThe answer is that the cells are epigenetically different. To understand this, it is first essential to clarify the central dogma in molecular biology. Your genome consists of about three billion base pairs of DNA, containing about 25,000 genes. To make a protein from a gene, enzymes bind to the gene and ‘transcribe’ it into an intermediate called mRNA. A second set of enzymes then ‘translate’ the mRNA into protein.
Some of the proteins encoded by those 25,000 genes, such as ones involved in cellular respiration, are essential to life, and must be continuously produced in every cell. However, most proteins are not expressed in all cells. As cells in an embryo become specialised to form tissues such as brain or liver, they permanently alter their patterns of gene activity, and so produce different proteins, to take on the characteristics of brain or liver cells. Epigenetics manifests these great changes in gene activity by altering the accessibility of genes to the enzymes involved in transcription.
Research suggests that epigenetics can confer some cellular ‘memory’ of past stress, and that changes
in the epigenome may even be transmitted between generations, though this is still controversial. The go-to example for epigenetics researchers is the Dutch Hunger-Winter of 1944-1945. This was a deadly famine in the Netherlands lasting three months, precipitated by a German blockade, and worsened by an unusually harsh winter. It was a tragedy, but presented researchers with a unique ‘natural experiment’. It was found that babies born to Dutch women who had good access to food during the initial six months of pregnancy, but starved for the last three months, were underweight, whereas mothers starved during the first three months but well fed during the final six gave birth to babies of healthy weight. This is logical, considering that most foetal growth occurs during the final months of pregnancy. More surprising was data showing that the babies born underweight had, on average, a substantially lower incidence of obesity than the general population for the remainder of their lives, whereas those malnourished early in gestation, but born at a healthy weight, had a higher incidence of obesity. These same effects were even observed to occur in the subsequent generation, decades after the original famine.
Epigenetics can explain this environmentally induced, but heritable, difference between the weights of these two groups. It has been shown that those individuals starved early in gestation had comparatively reduced methylation of the gene encoding an insulin-like growth factor (IGF2), and so higher levels of the protein. This protein is a hormone that promotes glucose metabolism in a similar way to insulin. In short, these individuals have developed a ‘thrifty’ metabolism.
 To find out about current epigenetics research, we spoke with Professor Wolf Reik, associate director of one of the largest institutes of epigenetic research in Europe, the Babraham Institute. One of the most exciting recent developments in epigenetics, actively researched in Professor Reik’s lab, is technology for editing the epigenome. This makes use of the revolutionary CRISPR-Cas9, originally a remarkably precise technique for cutting DNA, modified to alter epigenetic marks. Though still is in its infancy, CRISPR-Cas9 and the modulation of the epigenome have the potential to be used in the treatment of a vast range of diseases, including cancer.
To find out about current epigenetics research, we spoke with Professor Wolf Reik, associate director of one of the largest institutes of epigenetic research in Europe, the Babraham Institute. One of the most exciting recent developments in epigenetics, actively researched in Professor Reik’s lab, is technology for editing the epigenome. This makes use of the revolutionary CRISPR-Cas9, originally a remarkably precise technique for cutting DNA, modified to alter epigenetic marks. Though still is in its infancy, CRISPR-Cas9 and the modulation of the epigenome have the potential to be used in the treatment of a vast range of diseases, including cancer.Cancer is caused by aberrant activity of the genes controlling cell division, in some cases arising from epigenetic mutations. Changing the epigenetic marks on these regulatory genes could therefore be a target of novel cancer therapies. Promisingly, epigenetic silencing of the gene SOX2 in human breast cancer cells grafted into living mice (a good model for breast cancer) caused inhibition of tumour growth for more than one hundred days. Nevertheless, success in mice is a far stretch from success in patients, Professor Reik also cautions that most cancers involve so many genetic and epigenetic changes that it would be difficult to halt a tumour’s progress by epigenetically modifying a single gene, except in the very early stages.
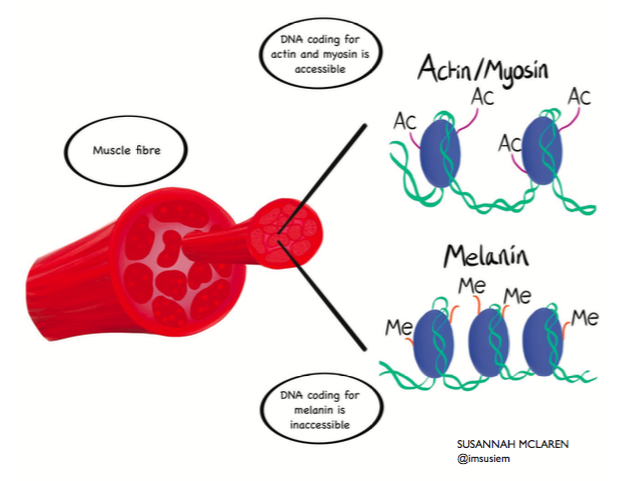 Accessibility of DNA determines what genes are active in body tissues. In muscle (pictured), genes encoding the muscle-specific proteins actin and myosin are in 'open' configuration via the addition of acetyl groups (-Ac) to the DNA packing proteins. In contrast, the skin-specific gene melanin is 'switched off' because the methyl groups (-Me) attached to the DNA render it inaccessible.
Accessibility of DNA determines what genes are active in body tissues. In muscle (pictured), genes encoding the muscle-specific proteins actin and myosin are in 'open' configuration via the addition of acetyl groups (-Ac) to the DNA packing proteins. In contrast, the skin-specific gene melanin is 'switched off' because the methyl groups (-Me) attached to the DNA render it inaccessible.of different genes. With this understanding would come the ability to turn genes on and off at will. With that power, could we wipe the epigenome clean to generate stem cells? Could we program stem cells to develop into nerve cells for the paralysed, or pancreatic cells for the diabetic, or retinal cells for the blind?
If we could understand how the epigenome changes with age, could we even reverse ageing?
Doubtless, these prospects are all a long way off, and a lot of basic science is needed before we consider them too seriously.
Some question the ethics of editing the epigenome in this way. Is it not just as contentious as genome editing, associated with designer babies (or worse)? Professor Reik’s view is nuanced, but overall, he believes that epigenome editing ‘is not playing God’. Reik argues that epigenetic changes are not permanent – with exceptions (such as IGF2), they are generally thought to last only for one generation, before the slate is wiped when sperm and egg cells are generated. Epigenetic changes are therefore more similar to conventional medicines in that they affect only the individual consenting to the treatment.
We are not altering the hereditary code which ‘makes us human’. However, we are potentially changing that individual’s life for the better. Could this be the middle ground we have all been waiting for?
