MONDAY, 3 MAY 2010
To diagnose a problem or identify a mechanism, one must first find evidence for some hypothesis; something one can test for that will prove or disprove the theory. In many scientific fields this evidence is provided by the presence of biological markers, or biomarkers.The most basic definition of a biomarker is simply a substance that can be used as a sign of an organism’s genetic or physical state. They are used in scientific fields from psychiatry, where they can help to find a genetic basis for illnesses such as schizophrenia, to geology and astrobiology, where ‘biosignatures’ point to the existence of living organisms in the past or present.
However, it is in medicine and medical research where biomarkers are most commonly used. In these fields the term denotes biological traits that can identify the progress of a disease or condition. Hormones, enzymes, specific cells, antibodies, and genes can all be biomarkers. Physical changes such as tumour growth can also be considered biomarkers in medicine. These markers can be measured by a variety of methods, including physical examination (checking suspect lumps is an example), laboratory testing (of blood samples, for instance), and medical imaging by systems such as X-rays or magnetic resonance imaging.
In research, biomarkers often refer to genetic markers – fragments of DNA that are often mutated or altered in specific diseases. Researchers who want to learn about the development of a disease and its response to novel treatments are able to add a detectable tag to these fragments of DNA so the resultant proteins and cells in which they are expressed can be distinguished. Their behaviour or progression during disease development or after therapy can then be studied. To advance our understanding of the biological processes involved, it is desirable to examine them as they occur in living animals (in vivo), so imaging strategies have been developed to track biomarkers that allow monitoring in real time.
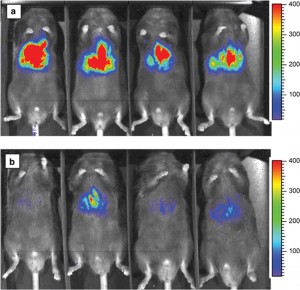
One such tagging system is bioluminescence imaging (BLI), which takes advantage of a process found in nature. Organisms such as the North American firefly, and many marine creatures, emit a light known as bioluminescence. These organisms use it for various reasons, such as a disguise, to lure prey, in mating rituals, or as a reaction to stress. It is produced by a chemical reaction within the organism where an enzyme, luciferase, oxidises a substrate, luciferin. This reaction produces oxyluciferin, along with the release of photons of light.
Genetically engineered cells that express the luciferase enzyme (for example tumour cells) can be introduced into an animal model, and when luciferin is admitted into the bloodstream the light emitted from the engineered cells can be detected using cameras. Since mammalian cells do not emit light and no external stimulus is required, BLI allows sensitive detection and quantification of only those cells specifically engineered to exhibit bioluminescence. It thus provides low-cost, non-invasive and real-time analysis of disease processes at the molecular level in a single living animal. This is a more desirable strategy than traditional procedures, which involve analysing many animals to accurately gauge what is happening at different stages of the disease.
The most common use for BLI has been the non-invasive detection of tumours and quantification of their growth and progression. Since genetic engineering is necessary to label the cells of interest, it is unlikely to be used directly in human studies. However, animal studies using BLI will be useful in predicting the human response to therapies in the early stages of development during pre-clinical trials.
BLI has also been used to track the spread of bacterial and viral infections in animal models and is shedding light on the processes that lead to neurodegenerative disease such as Alzheimer’s. Such studies will greatly advance the analysis of a wide range of diseases, making the new technology of bioluminescence imaging and its ability to track biomarkers an important innovation.
Lindsey Nield is a PhD student in the Department of Physics